When you pick up a prescription, do you know whether your pharmacist is required to swap your brand-name drug for a cheaper generic - or just allowed to? The answer depends entirely on which state you live in. This isn’t just a paperwork detail. It affects how much you pay, whether you stick to your treatment plan, and even whether your medication works as intended. In some states, pharmacists must substitute generics unless told otherwise. In others, they can’t swap anything without your explicit permission. These differences aren’t minor. They’ve been shown to change how often generics are actually used - by as much as 20 percentage points.
What’s the Real Difference Between Mandatory and Permissive Substitution?
Mandatory substitution means your pharmacist must give you the generic version of a drug if it’s available and approved by the FDA - unless your doctor specifically says not to. Think of it like a default setting: substitution happens automatically, and the only way to stop it is by writing "Dispense as Written" or "Brand Medically Necessary" on the prescription.
Permissive substitution is the opposite. Here, pharmacists are allowed to substitute generics, but they don’t have to. They can choose to give you the brand name, even if the generic is cheaper and equally effective. In these states, the decision often comes down to the pharmacist’s judgment - or whether they even bring up the option.
As of 2020, 19 states had mandatory substitution laws. That includes Alabama, Alaska, Arizona, Arkansas, Colorado, Connecticut, Delaware, Idaho, Indiana, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Mississippi, Montana, New Hampshire, North Dakota, and West Virginia. The rest operate under permissive rules. But even within those two categories, there are layers of rules that change how substitution actually works in practice.
The Four Hidden Rules That Control Whether You Get a Generic
It’s not just about whether substitution is mandatory or permissive. Four other state-level rules shape what happens when you walk into the pharmacy:
- Notification requirements - Does the pharmacist have to tell you they switched your drug? 31 states and Washington, D.C. require this - even if the generic label already says so. That means they might hand you a slip, call you, or ask you to sign something.
- Consent protocols - Do you have to say "yes" before they swap? Seven states plus D.C. require explicit patient consent. That means they can’t just assume you’re okay with it. You have to agree, in writing or verbally.
- Liability protections - If something goes wrong after the switch, is the pharmacist protected? In 24 states, they’re not. That means they could be sued if you have a bad reaction, even if the generic is FDA-approved.
- Prescriber restrictions - Can your doctor block substitution? All states allow this, but some require doctors to justify why they’re blocking it. Others let them just write "Do Not Substitute" without explanation.
These rules combine to create wildly different experiences. For example, in a state with mandatory substitution and no consent requirement, nearly all prescriptions for drugs like simvastatin were filled with generics within six months of the brand going off-patent. But in states that required patient consent, less than one-third of prescriptions were filled with generics - even though the generic was just as safe and effective.
Why This Matters: Real Numbers, Real Savings
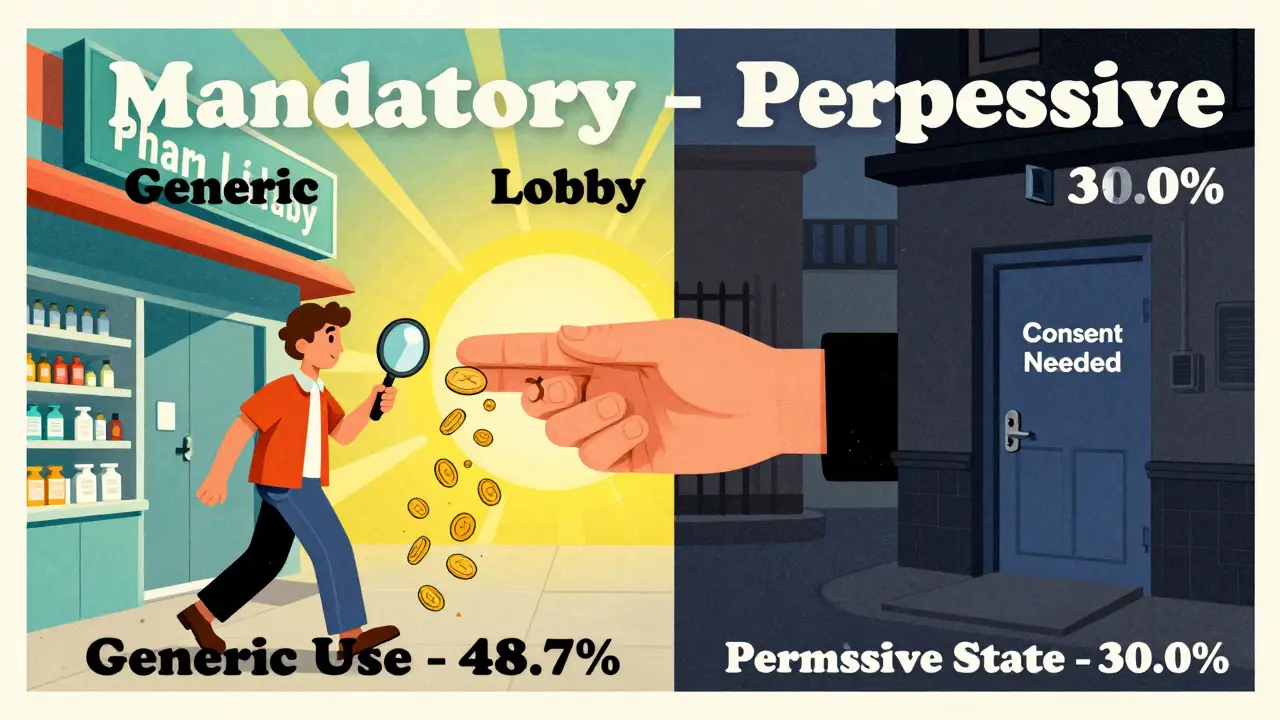
The impact isn’t theoretical. A 2011 study tracked simvastatin use across states. In mandatory substitution states, 48.7% of prescriptions were filled with the generic version. In permissive states? Only 30.0%. That’s an 18.7 percentage point gap - and it’s not just about money.
Generics cost 80-85% less than brand-name drugs. If just 1% more people used generics, Medicare Part D would save $160 million a year. Multiply that across Medicaid, private insurers, and out-of-pocket costs, and you’re looking at billions saved annually. But those savings don’t happen unless substitution actually occurs.
And here’s the kicker: states that required patient consent had the lowest generic use - just 32.1% of prescriptions filled with generics. That’s because many patients don’t know they’re being offered a choice. They assume the pharmacist is giving them what the doctor ordered. If they’re not told they can save money, they rarely ask.
What About Biosimilars? The New Frontier
Biosimilars - cheaper versions of complex biologic drugs like Humira or Enbrel - are changing the game. Unlike small-molecule generics, biosimilars aren’t exact copies. They’re highly similar, but there’s more uncertainty around switching back and forth between them.
Because of that, 45 states have stricter rules for biosimilars than for regular generics. Most require doctors to be notified before substitution. Some require patient consent. A few even require pharmacists to keep detailed records of every switch.
Only 9 states and D.C. treat biosimilars the same way they treat simple generics. That means if you’re on a biologic drug, your chances of getting a biosimilar depend heavily on where you live. In states with tight rules, substitution is rare - even when the biosimilar is cheaper and approved.
How Prescribers and Pharmacists Navigate the Patchwork
Doctors have to learn the rules of every state they prescribe in. A prescription written in California might be handled differently than one written in Texas. Some states use special two-line prescription pads - one line for the drug name, another for substitution approval. Others rely on phrases like "Do Not Substitute" written in the margin.
Pharmacists, meanwhile, face a logistical nightmare. In mandatory states, they must check every prescription for restrictions. In permissive states, they have to decide whether to suggest a switch - and then navigate consent and notification rules. In states without liability protection, they may avoid substitution altogether, even if it’s legal, just to stay safe.
And if you’re on a narrow therapeutic index drug - like warfarin, levothyroxine, or phenytoin - the stakes are higher. Even tiny differences in absorption can cause serious side effects. That’s why some pharmacists hesitate, even in mandatory states. But studies show that when substitution is allowed without consent, pharmacists are more likely to switch these drugs safely - because they’re not stuck in legal gray areas.
What You Can Do: Know Your Rights
Here’s the bottom line: you have more control than you think.
- If you’re on a generic and your pharmacy switches you back to the brand without asking - that’s legal in permissive states, but you can ask why.
- If you’re being charged more and you don’t know why - ask if a generic is available. You might be surprised.
- If your pharmacist doesn’t mention substitution, don’t assume they’re not allowed to. In permissive states, they’re allowed - they just don’t have to tell you.
- Always check your prescription label. If it says "Substitution Not Allowed," your doctor blocked it.
- Ask your doctor to write "Dispense as Written" only if you truly need the brand. Otherwise, leave it open.
There’s no national standard. That means your pharmacy experience can change if you move. If you’re switching states, check your new state’s pharmacy board website. Most have a section on generic substitution laws.
Why This System Exists - And Why It’s Changing
The U.S. didn’t create this mess by accident. The Hatch-Waxman Act of 1984 set up the modern generic drug approval system but left substitution rules to the states. That was meant to give states flexibility. Instead, it created a patchwork that’s confusing for everyone: patients, doctors, pharmacists, and insurers.
But the trend is clear. Between 2014 and 2020, the number of mandatory substitution states rose from 14 to 19. More states are moving toward default substitution - because the data shows it works. More generics mean lower costs, better adherence, and fewer hospitalizations.
Still, the pharmaceutical industry pushes back. Brand-name drugmakers spend millions lobbying in permissive states to keep patient consent rules strong. They know that if patients don’t know they’re being offered a cheaper option, they’ll keep paying more.
As biosimilars become more common, this battle will only get louder. But the science is clear: when substitution is easy and automatic, people use it. When it’s blocked by paperwork or consent forms, they don’t.
What’s Next?
Expect more states to adopt mandatory substitution with minimal consent requirements. The cost savings are too big to ignore. More states may also require pharmacists to proactively offer generics - not just allow it.
But until then, you’re the best advocate for your own care. Know the rules in your state. Ask questions. Don’t assume the pharmacist knows what you want. And if you’re paying more than you should - speak up. It’s not just about money. It’s about getting the right treatment, without unnecessary barriers.
Can my pharmacist switch my brand-name drug to a generic without telling me?
In mandatory substitution states, yes - as long as your doctor didn’t write "Dispense as Written." But 31 states and D.C. require pharmacists to notify you separately, even if the label says it’s generic. In permissive states, they can switch it, but they’re not required to tell you unless you ask.
Do I have to give permission before my pharmacist substitutes a generic?
Only in 7 states plus Washington, D.C. In those places, the pharmacist must get your verbal or written consent before switching. In the other 43 states, they can substitute without asking - especially if substitution is mandatory.
Why does my pharmacy sometimes give me the brand drug even when a generic is available?
It could be because your doctor wrote "Do Not Substitute," or your state has permissive substitution rules. It could also be that the brand is priced the same as the generic - in which case, substitution isn’t required even in mandatory states. Or, in some cases, the pharmacist may be cautious due to liability concerns.
Are biosimilars treated the same as regular generics?
No. 45 states have stricter rules for biosimilars. Most require doctor notification or patient consent before substitution. This is because biosimilars are more complex than small-molecule generics, and regulators are being cautious about switching between them.
How can I find out what substitution rules my state has?
Visit your state’s Board of Pharmacy website. Most have a section on generic substitution laws. Look for terms like "mandatory substitution," "patient consent," or "dispense as written." You can also call them directly - they’re required to answer public questions.